Dr. Ken Nicholas


Catalytic Conversion of Biomass-Derived Polyols to Chemicals and Fuels
Dr. Nicholas specializes in the area of organotransition metal chemistry, bridging the traditional disciplines of organic, inorganic and biological chemistry. The major theme of his research centers on the unique reactivity of transition metal-coordinated molecules. Nicholas seeks new understanding of this effect in chemical and biological systems, while also exploiting this effect for the invention of new reactions and their application in organic synthesis.
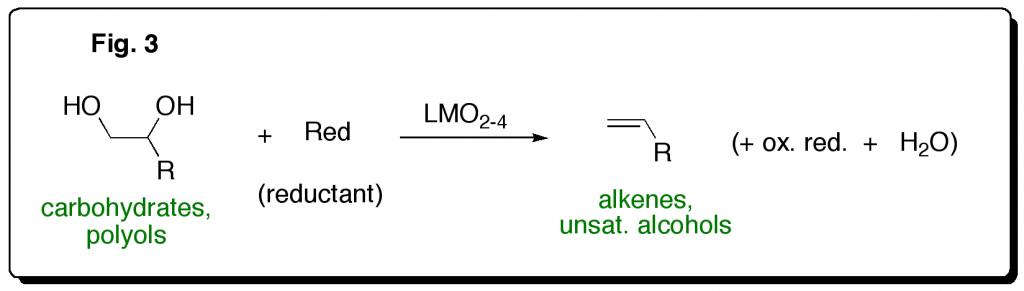
Biomass-derived carbohydrates and polyols are the most abundant renewable chemical feedstocks for potential conversion to fuels and value-added chemicals. Because of their high oxygen content relative to fuel products and many value-added chemicals, efficient and selective catalytic reactions are needed that remove oxygen, i.e. reductions, from these molecules. Following prior studies that demonstrated the ability of Cp*ReO3 to catalyze the deoxydehydration (DODH) of glycols to alkenes by phosphines (Fig. 3, below) the research team has shown that various oxo-rhenium (LReO3,4) complexes catalyze efficient polyol DODH by the more economical and benign reductant, sulfite (SO32-).
Current research efforts and goals include:
- The discovery of more robust, economical and heterogeneous catalysts
- The application of the DODH reactions to abundant carbohydrate-derived polyols
- The evaluation of other commercially viable reductants, e.g. H2 and CO
- The elucidation of the catalytic reaction intermediates through experimental and computational studies.
Garry Chapman | Graduate Research Assistant | Georgia, United States | [email protected]
Bold items indicate OK EPSCoR-supported research
- Ahmad, I., G. Chapman, and K. M. Nicholas (2011). "Sulfite-Driven, Oxorhenium-Catalyzed Deoxydehydration of Glycols." Organometallics 30(10): 2810-2818. doi: 10.1021/om2001662.
Access the publication
- Vkuturi, S., G. Chapman, I. Ahmad, and K. M. Nicholas (2010). "Rhenium-Catalyzed Deoxydehydration of Glycols by Sulfite." Inorganic Chemistry 49(11): 4744-4746. ACS Publications. doi: 10.1021/ic100467p.
Access the publication
- Penoni, A., G. Palmisano, G. Broggini, A. Kadowaki, and K. Nicholas (2005). "Efficient Synthesis of N-Methoxyindoles via Alkylative Cycloaddition of Nitrosoarenes with Alkynes." The Journal of Organic Chemistry 71(2): 823-825. doi: 10.1021/jo051609r.
Access the publication
- Zhou, L., D. Powell, and K. M. Nicholas (2006). "Tripodal Bis(imidazole) Thioether Copper(I) Complexes: Mimics of the Cu(B) Site of Hydroxylase Enzymes." Inorganic Chemistry 45(10): 3840-3842. ACS Publications. doi: 10.1021/ic052121b.
Access the publication
- Biswajit K. and K. M. Nicholas (2005). "Copper-Catalyzed Allylic Hydroxyamination and Amination of Olefins with BOC-Hydroxylamine", Tetrahedron, 46: 1451.
- Stephens, J. C., M. A. Khan, and K. M. Nicholas. (2005). "Cyclopentadienyliron Complexes of Nitrosobenzene: Preparation, Structure and Reactivity with Olefins." Journal of Organometallic Chemistry 690(21-22): 4727-4733. doi: 10.1016/j.jorganchem.2005.06.045.
Access the publication
- Srivastava, R. S. and K. M. Nicholas (2005). "Kinetics of the Allylic Amination of Olefins by Nitroarenes Catalyzed by [CpFe(CO)2]2." Organometallics 24(7): 1563-1568. doi: 10.1021/om049336y.
Access the publication
- Srivastava, R. S., M. A. Khan, and K. M. Nicholas (2005). "Nitrosoarene−Cu(I) Complexes Are Intermediates in Copper-Catalyzed Allylic Amination." Journal of the American Chemical Society 127(20): 7278-7279. doi: 10.1021/ja044093m.
Access the publication
Toward Sustainable Chemicals and Fuels: Catalytic Conversion of Biomass Polyols to Olefins by Ken Nicholas
A presentation in the OK EPSCoR Biofuels Teleconference Series
